MDSC (engl.)
Myeloid-derived suppressor cells (MDSCs)
Myeloid-derived suppressor cells (MDSCs)
MDSCs formely also called myeloid suppressor cells (MSCs) were renamed in 2006 due to confusion with mesenchymal stem cells (also MSCs)(1).
MDSCs were originally identified in tumor bearing mice (2) and scarcely in chronic inflammatory or infectious diseases (3). Most descriptions of MDSCs refer to them by expression of the markers Gr-1 and CD11b and the statement that they represent "a hetergeneous population of immature myeloid cells" (4, 5). Since the Gr-1 and CD11b markers are also expressed by eosinophils and differentiated neutrophils (6) more refined analyses are required to define MDSCs.
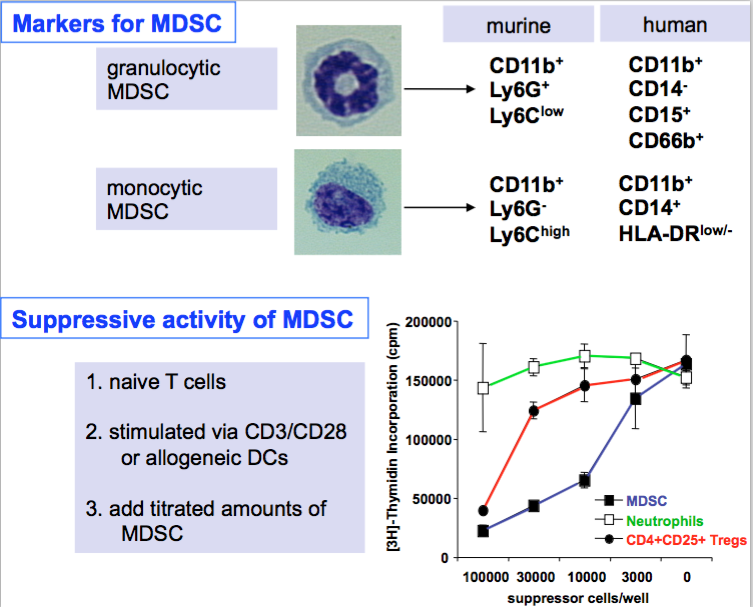
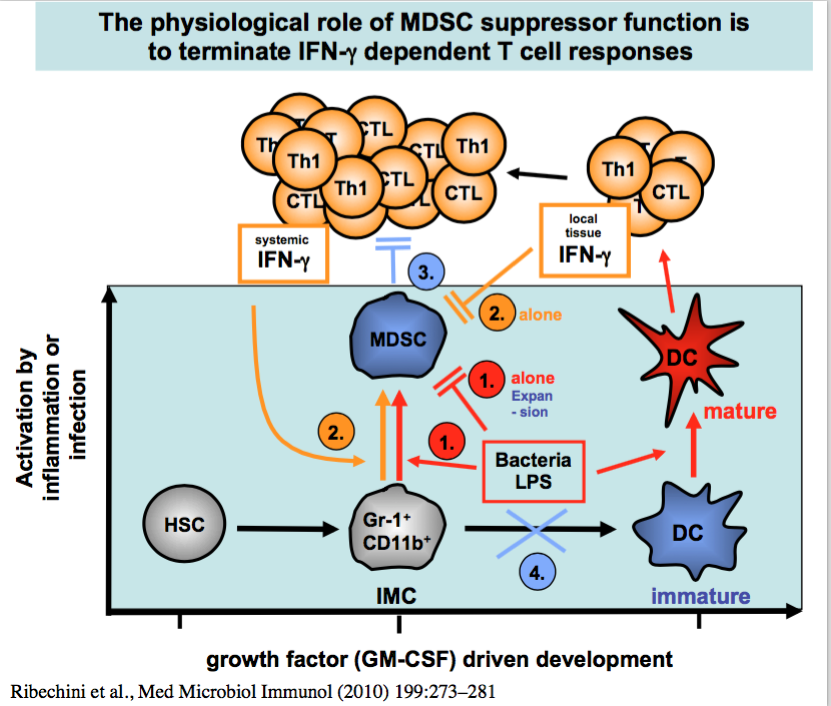
In mice and later in humans two subsets have been described from tumor-bearing mice (7, 8) but the same cells we could sort from spleens of healthy mice based on quantitative differences in Gr-1 and CD11b marker expression (6) or by the criteria shown in the figure below that include morphology (H&E staining), surface markers and - most importantly - suppressive capacity!
Our MDSC research
Description of a protocol to generate MDSCs in vitro
Our group was the first that described a protocol for the in vitro generation of murine MDSCs from bone marrow precursor cells using GM-CSF in a three day culture protocol (9).
Depletion of MDSCs by Gr-1 antibody
We also found that the in vivo use of Gr-1 (Rb6-8C5 clone) recognizing epitopes on the Ly-6C and Ly-6G molecules is limited for depletion experiments only under inflammatory conditions but not in healthy mice (10). The Gr-1 antibody remained for at least 4 days on the cell surface. Monocytic cells showed STAT1, STAT3 and STAT5 phosphorylation after Ly-6C ligation and were induced to differentiate transiently into macrophages. Induction of apoptosis by Gr-1 through Ly-6G of pre-neutrophils was prevented, most likely by their expression of the Bcl-2 family member Mcl-1. MDSC suppressor activity was only transiently interrupted for both subsets (10).
Activation conditions for MDSCs to become NO-secreting suppressors
The method to generate MDSC from non-suppressive precursor cells in vitro enabled us to investigate the activation requirements of MDSC. We found that only combined signals through IFN-g and LPS led to suppressor activity, while both factors alone were unable to do so (6).
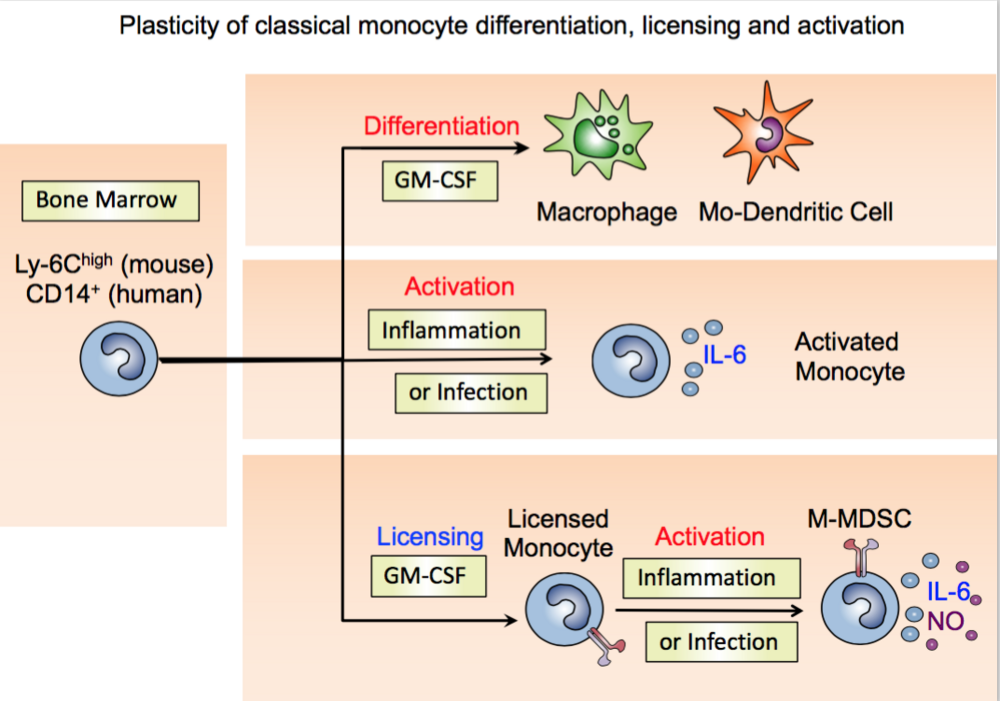
Monocyte licensing by known and novel GM-CSF signaling pathways is a pre-requisite for monocytic MDSC (M-MDSC) activation
Freshly isolated murine monocytes are unable to secrete NO and suppress T cells after LPS/IFN-g stimulation, while after 3 days of GM-CSF culture such monocytes do release NO to suppress T cells. Thus, monocytes require GM-CSF licensing to become M-MDSCs. With this initial finding we further analyzed the signaling pathways that are relevant for monocyte licensing. We found that the IRF-1 and PI3K > Akt > mTOR signaling pathways are activated in monocytes by GM-CSF and necessary for human and mouse M-MDSCs to develop suppressor function. Thus, both transcriptional and translational check points direct the development of monocytes towards M-MDSCs. Interestingly, human M-MDSCs secrete IDO instead of NO after activation of the very same signaling pathways to suppress T cells (11).
MDSCs are induced by mycobacteria
Together with the Walzl/Du Plessis-group in South Africa we found that Mycobacterium tuberculosis (Mtb) patients show increased frequencies of MDSC in their blood (12, 13).
Current Projects:
- We further investigate the interaction of mycobacteria with MDSCs for their activation (FACS, confocal microscopy, gene-deficient mice).
- MDSC generation in mice during Mycobacterium bovis BCG infection (RNA sequencing technologies).
- In vivo homing of murine MDSCs and functional investigation of novel homing markers.
- Identification of novel MDSC markers and suppressor mechanisms (RNA sequencing technologies).
References
1. Gabrilovich, D. I., V. Bronte, S. H. Chen, M. P. Colombo, A. Ochoa, S. Ostrand-Rosenberg, and H. Schreiber. 2007. The terminology issue for myeloid-derived suppressor cells. Cancer research 67: 425; author reply 426.
2. Bronte, V., M. Wang, W. W. Overwijk, D. R. Surman, F. Pericle, S. A. Rosenberg, and N. P. Restifo. 1998. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J. Immunol. 161: 5313-5320.
3. Serafini, P., C. De Santo, I. Marigo, S. Cingarlini, L. Dolcetti, G. Gallina, P. Zanovello, and V. Bronte. 2004. Derangement of immune responses by myeloid suppressor cells. Cancer immunology, immunotherapy : CII: 64-72.
4. Gabrilovich, D. I., and S. Nagaraj. 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews. Immunology 9: 162-174.
5. Gabrilovich, D. I., S. Ostrand-Rosenberg, and V. Bronte. 2012. Coordinated regulation of myeloid cells by tumours. Nature reviews. Immunology 12: 253-268.
6. Greifenberg, V., E. Ribechini, S. Rossner, and M. B. Lutz. 2009. Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur. J. Immunol. 39: 2865-2876.
7. Youn, J. I., S. Nagaraj, M. Collazo, and D. I. Gabrilovich. 2008. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 181: 5791-5802.
8. Movahedi, K., M. Guilliams, J. Van den Bossche, R. Van den Bergh, C. Gysemans, A. Beschin, P. De Baetselier, and J. A. Van Ginderachter. 2008. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111: 4233-4244.
9. Rößner, S., C. Voigtländer, C. Wiethe, J. Hänig, C. Seifarth, and M. B. Lutz. 2005. Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro. Eur. J. Immunol. 35: 3533-3544.
10. Ribechini, E., P. J. Leenen, and M. B. Lutz. 2009. Gr-1 antibody induces STAT signaling, macrophage marker expression and abrogation of myeloid-derived suppressor cell activity in BM cells. Eur. J. Immunol. 39: 3538-3551.
11. Ribechini, E., J. Hutchinson, S. Walter, U. Schleicher, A.-L. Jordán Garrote, S. J. Potter, N. Müller, H. Raifer, M. Huber, A. Beilhack, M. Lohoff, C. Bogdan, H. M. Hermanns, E. K. Geissler, and M. B. Lutz. 2017. Novel GM-CSF signals via IFN-gR/IRF-1 and AKT/mTOR license monocytes for suppressor function. Blood Advances 1: 947-960.
12. du Plessis, N., L. Loebenberg, M. Kriel, F. von Groote-Bidlingmaier, E. Ribechini, A. G. Loxton, P. D. van Helden, M. B. Lutz, and G. Walzl. 2013. Increased Frequency of Myeloid-derived Suppressor Cells during Active Tuberculosis and after Recent Mycobacterium tuberculosis Infection Suppresses T-Cell Function. Am J Respir Crit Care Med 188: 724-732.
13. Du Plessis, N., R. Jacobs, A. Gutschmidt, Z. Fang, P. D. van Helden, M. B. Lutz, A. C. Hesseling, and G. Walzl. 2017. Phenotypically resembling myeloid derived suppressor cells are increased in children with HIV and exposed/infected with Mycobacterium tuberculosis. Eur J Immunol 47: 107-118.