Project P06
P06 Optimizing immune cell-based protection against CMV in preclinical models
Prof. Dr. Reinhold Förster
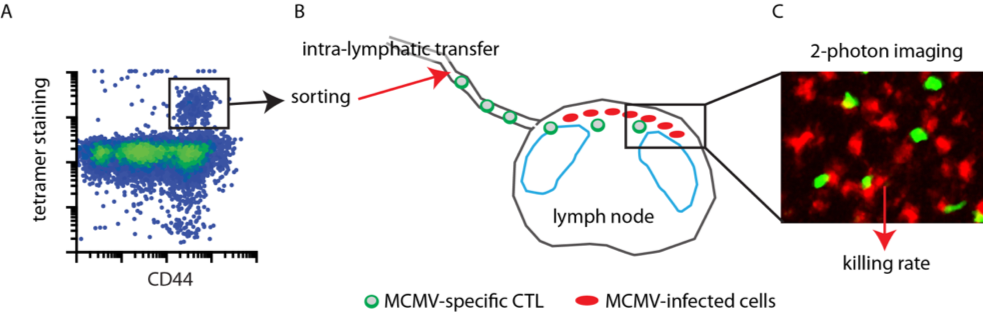
Immune cells that can eliminate virus-infected or tumour cells are used in clinical trials to better protect human patients from viral diseases or cancer (1, 2). Cytotoxic T lymphocytes (CTL) and natural killer (NK) cells protect against cytomegalovirus (CMV) disease in preclinical animal models and first in man studies. However, it remains unclear how efficiently single immune cells protect the host. It is also unclear whether cell-cell interactions and direct killing of CMV-infected cells are the most important component of the antiviral clinical benefit, or whether additional cytokine-mediated effects also substantially contribute to protection. Thus, it remains difficult to predict how to best protect against CMV reactivation after e.g. stem cell transplantation, because the most protective cell types and the number of cells needed to achieve clinical relevant level of virus control remain elusive. We recently developed an in vivo imaging approach that allows the direct observation and quantification of immune cells attacking mouse cytomegalovirus-(MCMV)-infected cells in vivo (3–5). With this relevant in vivo approach at hand, we aim to improve adoptive immunization protocols in a preclinical model that allows the generation of highly efficient cytotoxic immune cells to further foster knowledge-based T cell therapeutic approaches in a preclinical mouse model (Fig. 1). Thus, this project aims to reveal mechanisms for increasing immune cell-mediated target cell killing efficiency in a pre-clinical mouse model of MCMV infection. In the long run, these data will aid the establishment of an effective cellular therapy of CMV infection in humans and will support CMV-vaccine design, to better protect human patients from CMV-induced disease.
References
- Roddie, C., and K. S. Peggs. 2017. Immunotherapy for transplantation-associated viral infections. J. Clin. Invest.
- Mikucki, M. E., D. T. Fisher, J. Matsuzaki, J. J. Skitzki, N. B. Gaulin, J. B. Muhitch, A. W. Ku, J. G. Frelinger, K. Odunsi, T. F. Gajewski, A. D. Luster, and S. S. Evans. 2015. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat. Commun. 6: 7458.
- Halle, S., K. A. Keyser, F. R. Stahl, A. Busche, A. Marquardt, X. Zheng, M. Galla, V. Heissmeyer, K. Heller, J. Boelter, K. Wagner, Y. Bischoff, R. Martens, A. Braun, K. Werth, A. Uvarovskii, H. Kempf, M. Meyer-Hermann, R. Arens, M. Kremer, G. Sutter, M. Messerle, and R. Förster. 2016. In Vivo Killing Capacity of Cytotoxic T Cells Is Limited and Involves Dynamic Interactions and T Cell Cooperativity. Immunity.
- Zheng, X., S. Halle, K. Yu, P. Mishra, M. Scherr, S. Pietzsch, S. Willenzon, A. Janssen, J. Boelter, D. Hilfiker-Kleiner, M. Eder, and R. Förster. 2016. Cardiomyocytes display low mitochondrial priming and are highly resistant toward cytotoxic T-cell killing. Eur. J. Immunol.
- Halle, S., O. Halle, and R. Förster. 2017. Mechanisms and Dynamics of T Cell-Mediated Cytotoxicity In Vivo. Trends Immunol.