C. RNA-export
C. Characterization of foamy viral RNA-export
Regulation of the foamy viral RNA export
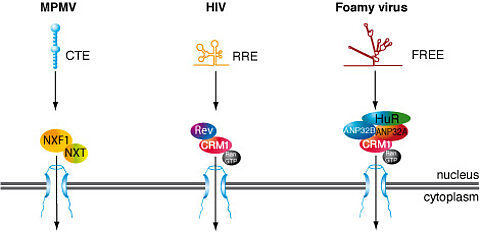
Most retroviruses express all their genes from a single primary transcript. In order to allow expression of more than one gene from this RNA, differential splicing is extensively used. Cellular quality control mechanisms retain and degrade non-spliced or partially spliced RNAs in the nucleus. Two pathways have been described how retroviruses circumvent this nuclear export inhibition. One, used by the Mason Pfizer Monkey virus involves a constitutive transport element (CTE) in the viral RNA that interacts with the cellular mRNA transporter-proteins NXF1/NXT1 to facilitate nuclear export (Figure 1). The other pathway is used by complex retroviruses such as HIV-1, MMTV and HTLV and relies on the recognition of a viral RNA element by a virus-encoded protein (HIV-1, Rev; HTLV, Rex; MMTV, Rem), which interacts with the karyopherin CRM1 (Figure 1).
We have analysed factors required for the nuclear export of non-spliced foamy virus mRNAs and described that this export is CRM1-dependent. In contrast to other complex retroviruses foamy viruses do not encode an export-mediating protein. We have shown that the cellular protein HuR binds to the foamy viral RNA export element (FREE). Our studies showed that both ANP32A and ANP32B, which are known to bridge HuR and CRM1, are essential for foamy viral RNA export. By using this distinct export pathway foamy viruses solve a central problem of viral replication.