Project P02
MCMV altered-self MHC-I molecules and their impact on cellular immune responses
Dr. Vanda Juranic Lisnic & Prof. Dr. Stipan Jonjic
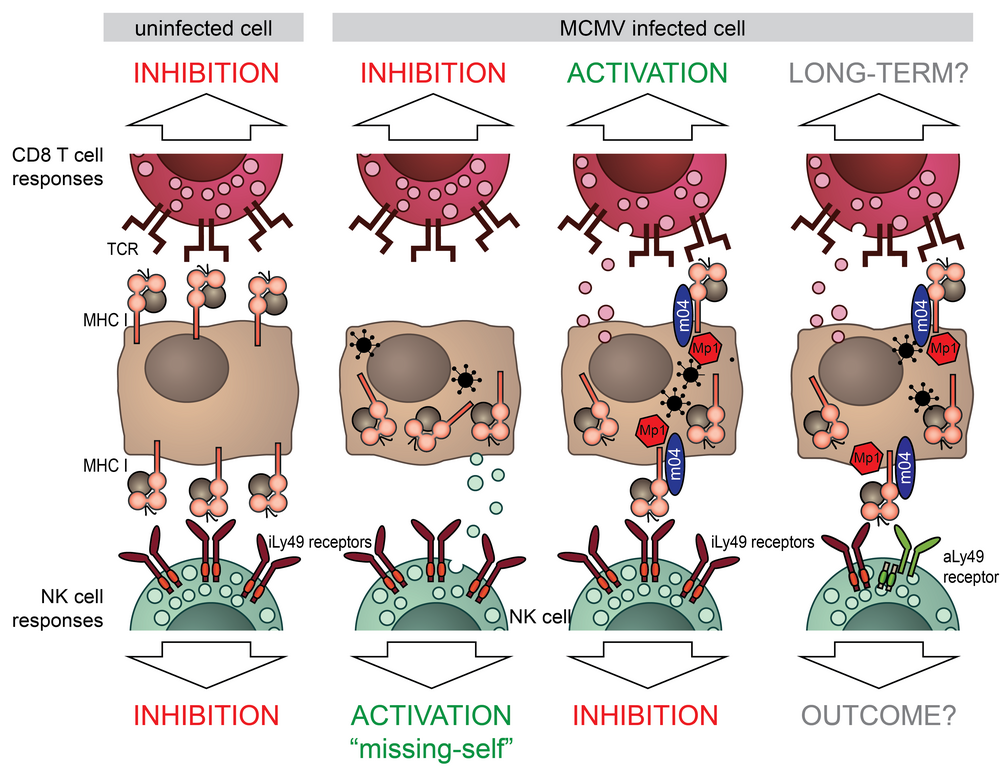
While down-regulating MHC-I from the surface protects the infected cells from cytotoxic T cells (CTLs), it also renders them sensitive to NK-cell-mediated “missing-self” attack. How CMVs balance between these two remains unclear. During the first funding period, we characterized a novel MCMV immunoevasin MATp1, which, together with the viral m04 protein, interferes with “missing-self” NK-cell activation. MATp1 and m04 collaborate to bring selected alleles of MHC-I to the cell surface in a trimolecular “altered-self” complex, which engages inhibitory Ly49 receptors more strongly than regular MHC-I molecules. This finding answered the long-standing question why licensed NK cells are incapable of controlling CMV infection. In addition to inhibitory Ly49 recognition, MCMV altered-self MHC-I molecules are specifically recognized by at least 3 activating Ly49 receptors; P, L and D2. This highlights the exceptional evolutionary pressure CMVs have exerted on the immune system. Furthermore, engagement of activating Ly49 receptors has been implicated in memory-like NK-cell responses.
We will investigate the long-term impact of MATp1/m04 on NK-cell and CTL responses. Our preliminary data suggests that MATp1 disrupts CTL responses, possibly by interfering with antigen presentation. MCMV has been an instrumental model for understanding CTL responses to HCMV. Furthermore, CMVs are promising vaccine vector candidates. Understanding how CMVs manipulate immune responses is a prerequisite for the development of novel therapeutical options and usage of CMVs as vectors. To that aim, we will employ high-throughput MHC-I-ligandome analysis and T-cell receptor (TCR) sequencing with in vivo and in vitro analyses of CTL responses. We will utilize several well-established adoptive transfer models to study the impact of MATp1/m04 on both CTL and NK-cell memory formation. As in the first funding period, we will extensively collaborate with other members of the RU and provide support for in vivo animal experiments and the development of novel monoclonal antibodies.