Project P07
P07 Role of NKG2D ligands for activation of the human cytomegalovirus specific immune response – perspectives for CMV vaccine development
Prof. Dr. Christine Falk & Prof. Dr. Martin Messerle
A vaccine protecting against CMV infection and CMV-associated disease would be of great medical benefit for several patient groups at risk. Boosting of CMV immunity in recipients of solid organ transplants before transplantation would reduce the risk for CMV reactivation and lower the incidence of acute and chronic graft rejection, hereby prolonging the functioning and survival of transplanted organs. Vaccination of recipients of hematopoietic stem cell transplants (HSCT) early upon immune reconstitution with a safe CMV vaccine to prevent CMV-associated impairment of HSC engraftment and immune reconstitution after HSCT. Another goal is the prevention of transmission of CMV from mother to the unborn child (congenital CMV infection), which is the leading viral cause of birth defects. Clinical testing of CMV vaccine candidates was thus far of limited success. The development of an effective and safe CMV vaccine is thus highly challenging.
There are several reasons that make it difficult to achieve this aim:
- The immune response induced in healthy individuals upon CMV infection – though able to clear the acute infection – cannot prevent establishment of latency, leaving the risk for reactivation and recurrence of infection.
- Through an arsenal of immunomodulatory functions (1) CMV can reactivate in the presence of adaptive immunity acquired after primary CMV infection, and re-infection with other strains is possible as well.
- Vaccination should therefore elicit immunity of superior quality than observed upon natural CMV infection.
- Several patient groups at risk of CMV disease have an impaired immune system and are highly susceptible to viral infections.
- A CMV vaccine for these patients thus needs to be highly attenuated, yet should nevertheless display strong immunogenicity.
NK cells and CD8+ T cells were found to be central in controlling CMV infection (1-5) and also, the humoral immune response is important to prevent dissemination of CMV within an organism. Consequently, we follow the hypothesis that an ideal CMV vaccine should be capable of inducing both innate (NK cells) as well as adaptive immunity (T cells, antibodies).
HCMV invests a lot of its coding potential to prevent NK cell activation, e.g. by expressing viral proteins that engage inhibitory receptors on NK cells as well as immune-evasins that prevent surface expression of ligands triggering activating NK cell receptors. Downregulation of HLA-I molecules from the surface of HCMV-infected cells makes these cells vulnerable to NK cell-mediated elimination. Furthermore, CMV influences the T cell response by expressing several HLA-I immune-evasins (e.g. US11), thereby preventing the priming of T cells to certain epitopes (called canonical MHC-I restricted epitopes), and re-directing the T cell response to non-canonical MHC‑I restricted epitopes.
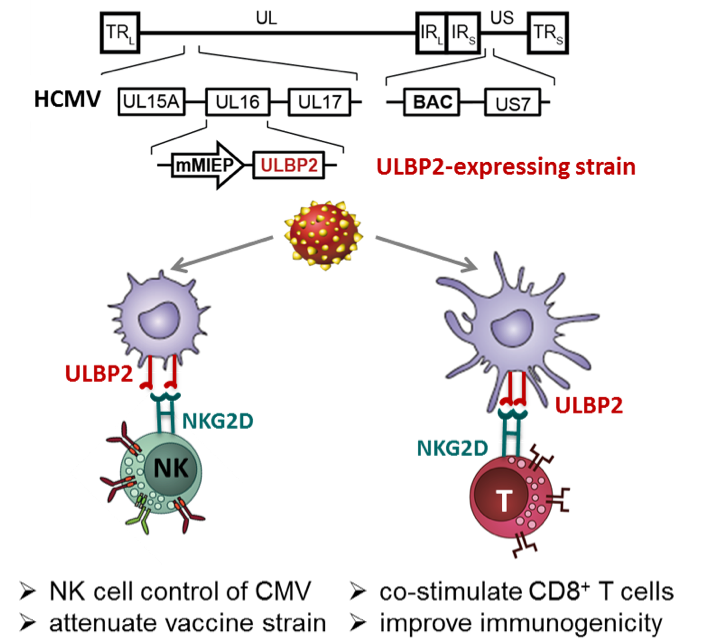
In the mouse model, we discovered that MCMV mutants expressing the RAE-1γ ligand for the activating NKG2D receptor of NK cells was robustly attenuated (6,7). Notably, the RAE-1γ-MCMV mutants preserved the ability to prime and maintain an effector T cell response, which was at least of the same quality and magnitude (partially even better) as the one detected after infection with wild-type MCMV (7). We have started to transfer this approach to HCMV and evaluate its utility for HCMV vaccine development (8).
In this project, we will investigate how human NKG2D ligands (such as ULBP2) expressed by HCMV mutants differ in their ability to activate NK cells, and how this affects NK cell-mediated control of viral spread. A second aim is to improve immunogenicity of CMV vaccine candidate strains by co-stimulation of CD8+ T cells via the NKG2D receptor and by fine-tuning antigen presentation in defining the optimal combination of HLA-I immune evasins.
References
- M. J. Reddehase, Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nat. Rev. Immunol. 2, 831-844 (2002).
- G. W. Wilkinson et al., Modulation of natural killer cells by human cytomegalovirus. J. Clin. Virol. 41, 206-212 (2008).
- C. S. Falk et al., NK cell activity during human cytomegalovirus infection is dominated by US2-11-mediated HLA class I down-regulation. J. Immunol. 169, 3257-3266 (2002).
- Q. Hammer et al., Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat. Immunol. 19, 453-463 (2018).
- J. Zischke et al., The human cytomegalovirus glycoprotein pUL11 acts via CD45 to induce T cell IL-10 secretion. PLoS Pathog. 13, e1006454 (2017).
- I. Slavuljica et al., Recombinant mouse cytomegalovirus expressing a ligand for the NKG2D receptor is attenuated and has improved vaccine properties. J. Clin. Invest 120, 4532-4545 (2010).
- T. Trsan et al., Superior induction and maintenance of protective CD8 T cells in mice infected with mouse cytomegalovirus vector expressing RAE-1gamma. Proc. Natl. Acad. Sci. USA 110, 16550-16555 (2013).
- A. Tomic et al., Activation of innate and adaptive immunity by a recombinant human cytomegalovirus strain expressing an NKG2D ligand. PLoS Pathog. 12, e1006015 (2016).