P01
Sphingomyelinase activation in T cells: Implications for T cell activation and paralysis
Sibylle Schneider-Schaulies
Institute for Virology and Immunobiology, University of Würzburg, Versbacher Str. 7, 97078 Würzburg, Germany
Overview:
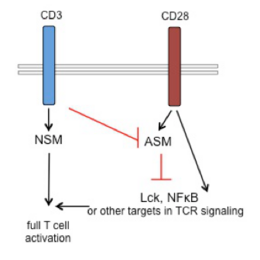
T cell paralysis is a major hallmark of measles virus (MV) induced generalized immunosuppression. We found that activation of the neutral, but not the acid sphingomyelinase takes part in physiological T cell co-stimulation in a timely and spatially restricted and coordinated transient manner. This is perturbed upon MV interaction with T cells, which causes prolonged activation of both NSM and ASM, and this appeared causatively linked to MV-induced loss of actin cytoskeletal dynamics in co-stimulated T cells. To identify molecular targets and pathways involved in MV deregulations of T cell activation at the level of sphingolipid dynamics, we first addressed the general role of sphingomyelinases in T cell activation and defined firstly, their importance in basal and stimulated metabolic T cell programming. Thus, pharmacological inhibition of NSM elevated general levels of both OXPHOS and glycolysis, and pharmacological inhibition of basal ASM activity prevented TCR stimulated oxidative phosphorylation (OXPHOS) bursting. Importantly, MV abrogated the TCR-induced OXPHOS burst, and and this was prevented by NSM inhibition. In the second funding period, we will identify cellular targets of NSM or ASM dependent regulation of OXPHOS versus glycolysis in co-stimulated T cells and their potential de-regulation by MV. Secondly, we found that NSM was required for CD3 signaling, and we will now address targets and mechanisms of NSM in CD3 signaling and their modulation by MV at the level of lipid and signalosome composition. The very same strategy will be employed for our third research focus: ASM activation by CD28 ligation alone appeared to prevent access of this co-stimulatory receptor to the TCR signaling machinery. Upon antigen recognition, signals emanating from the TCR licensed CD28 for co-stimulation by abrogating ASM activation. We will decipher mechanisms of TCR-mediated silencing of ASM activation by co-stimulating receptors. By this we aim to establish whether licensing versus silencing at the level of sphingomyelin breakdown and/or ceramide release is a general concept in co-stimulation and co-inhibition with the obvious goal to evaluate consequences if this is qualitatively de-regulated upon MV interaction.
References:
Original articles:
Gassert E, Avota E, Harms H, Krohne G, Gulbins E, Schneider-Schaulies S (2009) Induction of Membrane Ceramides: A Novel Strategy to Interfere with T Lymphocyte Cytoskeletal Reorganisation in Viral Immunosuppression. PloS Pathogens, 5(10): e1000623
Avota E, Gulbins E, Schneider-Schaulies S. (2011) DC-SIGN mediated sphingomyelinase-activation and ceramide generation is essential for enhancement of viral uptake in dendritic cells. Plos Pathogens. 10.1371/journal.ppat.1001290
Mueller N, Avota E, Collenberg L, Gulbins H, Schneider-Schaulies S (2014). The role of the neutral sphingomyelinase in physiological and measles virus (MV) mediated T cell suppression. PLoS Pathog. 2014 10(12):e1004574.
Collenburg L, Walter T, Burgert A, Mueller N, Seibel J, Japtok L, Kleuser B, Sauer M, Schneider-Schaulies S (2016) A functionalized sphingolipid analogue for studying redistribution during activation in living T cells. J. Immunol 196(9):3951-62
Walter T, Collenburg L, Japtok L, Kleuser B, Schneider-Schaulies S, Müller N, Becam J, Schubert-Unkmeir A, Kong JN, Bieberich E, Seibel J.(2016) Incorporation and visualization of azido-functionalized N-oleyl serinol in Jurkat cells, mouse brain astrocytes, 3T3 fibroblasts and human brain microvascular endothelial cells. Chem Commun (Camb). 52(55):8612-4.
Reviews:
Avota E, Gassert E, Schneider-Schaulies S (2011). Cytoskeletal dynamics: concepts in measles virus replication and immunomodulation. In: Viruses. Special Issue: Cytoskeleton in viral infections. Greber U, Sodeik B eds. 3(2), 102-117; doi:10.3390/v3020102
Schneider-Schaulies J, Schneider-Schaulies S. (2012): Viral Infections and sphingolipids. In ‚Sphingolipids in Disease’. Gulbins E, Petrache I eds. pp. 321-340.
Avota E, Koethe S, Schneider-Schaulies S (2013). Membrane dynamics and interactions in measles virus dendritic cell infections. Cell. Microbiol. 15(2):161-9.
Schneider-Schaulies S, Mueller N, Gulbins E (2014). Membrane Microdomains Enriched in Ceramides: From Generation to Function. In: Cell Membrane Nanodomains: From Biochemistry to Nanoscopy (p.134-172) Cambi & Lidke Eds., CRC Press, Taylor & Francis group ISBN 9781482209891
Avota E, Schneider-Schaulies S (2014) The role of sphingomyelin breakdown in measles virus immunomodulation. Cell. Physiol. Biochem. 34(1):20-6. doi: 10.1159/000362981. Epub 2014 Jun 16.
Schneider-Schaulies J, Schneider-Schaulies S (2014). Sphingolipids in viral infection. Biol Chem. 2014 Dec 19. pii: /j/bchm.just-accepted/hsz-2014-0273/hsz-2014-0273.xml. doi: 10.1515/hsz-2014-0273. [Epub ahead of print]