Project P04
P04 Impact of HCMV infection on professional antigen presenting cells
Prof. Dr. Britta Eiz-Vesper & Prof. Dr. Ulrich Kalinke
Healthy individuals infected with HCMV usually control the virus by cytotoxic CD8+ T cells without showing symptoms. In contrast, immunocompromised patients with low frequencies of functional HCMV-specific T cells may develop severe HCMV disease. In this case, the adoptive transfer of HCMV-specific T cells from third-party donors can improve anti-viral protection. To date, several hospitals use this approach as a standard of care. Nevertheless, low donor T cell frequencies as well as occasional ineffectiveness of the treatment despite the transfer of high numbers of HCMV-specific T cells are challenges which still need to be resolved to make this therapy available to all patients in need of it.
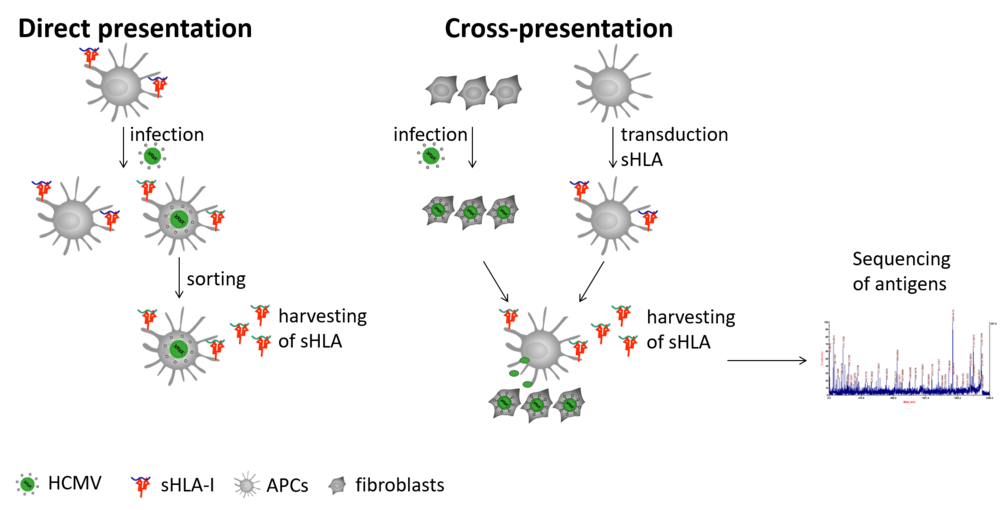
HCMV-specific T cells are primed by professional antigen presenting cells (APCs) presenting HCMV peptides in the context of HLA-I molecules. As HCMV modulates antigen presentation in infected cells, it is assumed that HCMV antigens are mainly presented by non-infected APCs by a process that is termed “cross presentation”. Since studies regarding HCMV antigen presentation mainly focused on non-immune cells, it is not yet well understood which HCMV antigens are presented by APCs, and in particularly the cross-presenting ones, to induce protective T cell responses. Moreover, every individual has a certain combination of six HLA-I alleles to present these antigens. However, these six HLA-I alleles not only differ between most individuals, but they also show different preferences in the antigens they present. Consequently, rather divergent HCMV-specific T cell responses are induced in single individuals. To improve adoptive T cell therapy, the mechanism of HCMV antigen presentation as well as the induction of T cell responses has to be better understood. Therefore, we aim to dissect host-to-host differences in antigen presentation by analyzing the HCMV antigens presented by directly and cross-presenting APCs of different individuals. We will transduce APCs with secreted HLA-I molecules (sHLA-I). In contrast to regular HLA-I molecules, these sHLA-I molecules are not membrane bound, but are secreted after antigen loading and can therefore be harvested from the cell supernatant in high amounts. Moreover, antigens from a distinct type of HLA-I allele can be analyzed. Additionally, the influence of different HLA-I allele combinations on antigen presentation and T cell responses will be investigated. A distinct focus will be laid on the analysis of how the variable TAP (transporter associated with antigen processing)-dependence of different HLA-I alleles to present peptides influences the antigen presentation. These approaches combined with the analysis of the respective induced T cell responses will help us to improve the generation of HCMV-specific T cell products for the treatment of patients.