Project P09
P09 Personalized medicine – Risk stratification and prevention of HCMV-related disease in transplant patients based on MHC-I-ligandomes
Hermann Einsele & Sabrina Kraus
HCMV reactivation or primary HCMV infection in immunocompromised patients still remains associated with severe clinical complications and considerable morbidity and also mortality despite improved and novel antiviral drug therapies (1, 2).
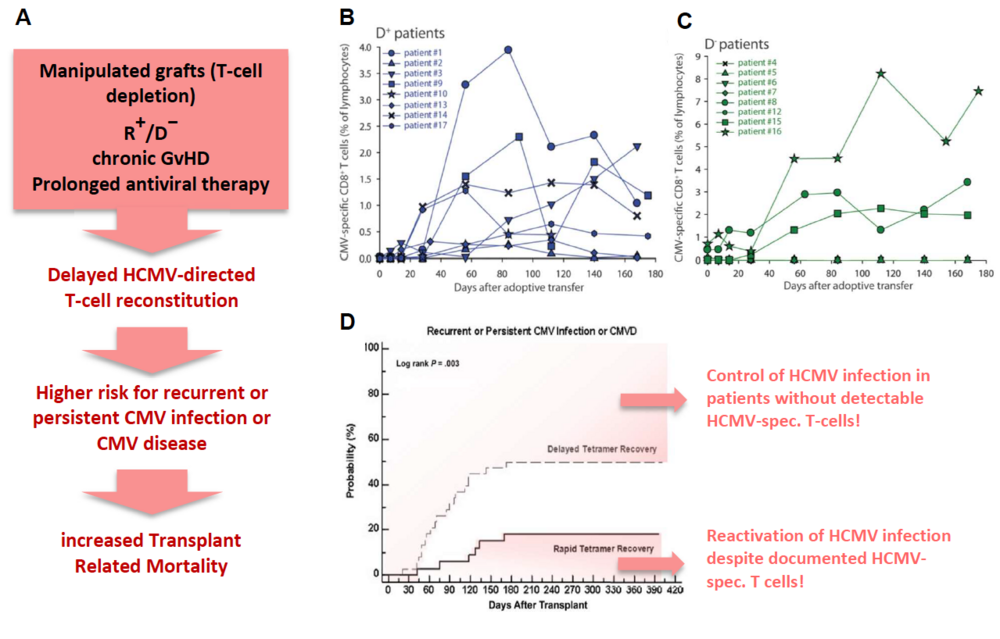
The risk of HCMV disease in these patients is determined by the magnitude and complexity of HCMV-specific immune responses, especially mediated by T and NK cells. Since full immune reconstitution of T cells takes at least two to three years, adoptive transfer of HCMV-directed donor-derived T and NK cells as well as vaccination strategies of donor and/or recipient are increasingly attractive therapeutic approaches to improve HCMV-directed immune reconstitution post-transplant. The GMP-graded isolation and the adoptive transfer of HCMV-specific T cells have successfully been applied (3, 4) (Fig.1B). Albeit T-cell transfer showed limited benefit for HCMV-seropositive patients egrafted from a seronegative donor. In most of these cases T cells are not traceable after transfer (Fig. 1C).
Fig. 1: Risk factors for HCMV disease after alloSCT (A, red box) and implications. Monitoring of adoptively transferred GMP-graded Streptamer-selected HCMV-specific CD8+-T cells in patients engrafted from a seropositive (B, (D+)) or seronegative (C, (D−)) donor (4). Probability of recurrent or persistent CMV infection after alloSCT in patients with delayed (dashed) and rapid (solid) T-cell (=Tetramer) recovery (7).
Several immunodominant HCMV-peptides, called FREPs (frequently recognized epitopes) have been shown to induce T-cell responses in alloSCT patients (5, 6). However, although HCMV-specific T-cells are detectable in a patient we are still unable to reliably predict if this patient will reactivate HCMV infection or will even need a long-lasting antiviral chemotherapy due to an insufficient HCMV-directed immune control. This insufficiency could result from an impaired T- and/or NK-cell reconstitution (caused by e.g. Graft-versus-Host-Disease=GvHD or antiviral prophylaxis), cell dysfunctions like T-cell exhaustion, anergy or senescence, and/or insufficient interaction between T or NK cells and the cells of innate immunity, like macrophages, due to a lack of necessary chemokines/cytokines or HCMV-specific antibodies triggering NK-cell mediated ADCC. On the other hand, some patients are able to control an HCMV infection without possessing detectable frequencies of HCMV-specific T-cells (7). In this case, the responsible T-cell epitopes have possibly either not been identified so far or disease control might be mediated primarily by adaptive NK cells that have been shown to play an important role in HCMV-control after transplant (8).
This project aims to better assess patients’ individual risk for developing chronic active HCMV infection following alloSCT by refining detection and monitoring of HCMV-specific T-cell immunity and reconstitution in alloSCT patients and their respective donors. We will improve selection and generation of HCMV-directed CD8+- and CD4+-T and NK cells as individually combined sets for personalized adoptive transfer and of HCMV-derived peptides for vaccination strategies. Moreover, we aim to better define the risk for GvHD development and to assess the influence of GvHD prophylaxis on HCMV-specific T-cell reconstitution. Reconstitution of antiviral T- and NK-cell responses, phenotype and functions will be studied in alloSCT patients, the respective stem cell donor and healthy donors using novel sets of HCMV epitopes that will be provided by this FOR (Z01, P01, P03, P04). Findings will be compared and correlated with the risk of HCMV reactivation and disease to better define epitopes that are crucial for controlling HCMV infection. We thereby aim to optimize immune monitoring, prediction of HCMV-related complications and improve adoptive T- and NK-cell therapies for patients at risk of HCMV infection as well as vaccination strategies for patients and donors.
References
- F. M. Marty et al., Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med 377, 2433-2444 (2017).
- R. F. Chemaly et al., Definitions of Resistant and Refractory Cytomegalovirus Infection and Disease in Transplant Recipients for Use in Clinical Trials. Clin Infect Dis 68, 1420-1426 (2019).
- C. Stemberger et al., Lowest numbers of primary CD8(+) T cells can reconstitute protective immunity upon adoptive immunotherapy. Blood 124, 628-637 (2014).
- M. Neuenhahn et al., Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia 31, 2161-2171 (2017).
- M. Odendahl et al., Clinical-scale isolation of 'minimally manipulated' cytomegalovirus-specific donor lymphocytes for the treatment of refractory cytomegalovirus disease. Cytotherapy 16, 1245-1256 (2014).
- G. U. Grigoleit et al., Dendritic cell vaccination in allogeneic stem cell recipients: induction of human cytomegalovirus (HCMV)-specific cytotoxic T lymphocyte responses even in patients receiving a transplant from an HCMV-seronegative donor. J Infect Dis 196, 699-704 (2007).
- J. W. Gratama et al., Immune monitoring with iTAg MHC Tetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: a prospective multicenter study. Blood 116, 1655-1662 (2010).
- F. Cichocki et al., Adaptive NK cell reconstitution is associated with better clinical outcomes. JCI Insight 4, (2019).