FOR2123
Sphingolipid dynamics in infection control
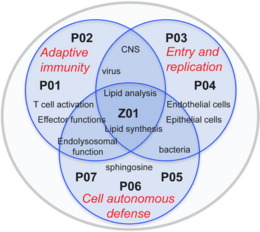
As major components of eucaryotic cell membranes, sphingolipids have the potential to impact on infection control at multiple levels. These include trapping, entry, compartmentalization and intracellular routing of pathogens themselves, but also lateral or vertical segregation of receptors and associated signaling complexes or recruitment or maturation of organelles. In addition, certain bioactive sphingolipids can regulate signaling pathways by themselves or act as bacteriocidals. This effectively regulates cell autonomous, innate and adaptive immune responses. Given this broad spectrum of activity, the highly dynamic metabolism of sphingolipids is tightly controlled under homeostatic conditions, and, when challenged by pathogen exposure, expectedly translates into major changes in host or immune cell physiology. The ultimate goal of FOR2123 is to identify novel targets and developing tools for (immuno) therapeutic interventions in disease. The scientific concepts of the consortium focus on the regulatory role of sphingolipid dynamics in two major aspects of pathogen control by addressing
1- activation and differentiation of T cell effector functions at a cellular level and in an infection model at the host, (P01, P02) and
2- uptake and trafficking of pathogens and cell autonomous defense mechanisms at the infected cell/organism level (P03 – P07)
with the vision to translate major findings into clinical application.
Bridging the research projects, the central service project Z01 provides novel unique analytical and technical tools and techniques to monitor dynamic redistribution of sphingolipids in the relevant host cell systems studied by the consortium.
Exploring the tools provided by the technical platform and overarching practical and theoretical approaches, common and complementary facets of sphingolipid dynamics in infection control are addressed in three major research areas.
1 Adaptive immunity:
Studies in this area address the general impact of sphingolipid turnover in the T cell compartment, how this is exerted and modified by measles virus (MV) in vitro and impacts on the control of MV in an experimental infection model (P01, P02).
2 Entry and replication:
Projects in this area address the role of sphingomyelinases, downstream enzymes and metabolites in Neisseria invasion into their respective target cells (P03, P04).
3 Innate/Cell autonomous defense:
This area investigates the role of sphingomyelinases in overcoming cell autonomous or host intrinsic control thereby assigning these enzymes an important role in the pathophysiology of BCG- or Staphylococcus aureus infections, respectively, and marking them as targets for interventive therapy (P05, P06). Moreover, the role of sphingosines, known to kill many bacterial species, in prevention of bacterial infections, is explored within area 3 (P07).